
CymaBay announced positive interim results from its ongoing low-dose Phase 2 study of seladelpar in patients with primary biliary cholangitis (PBC), a life-threatening and life-limiting chronic cholestatic liver disease. In the first part of the study, patients at high risk of disease progression, with an inadequate response to ursodeoxycholic acid (UDCA), as characterized by a persistent elevation in alkaline phosphatase (AP), or who were intolerant to UDCA, received either 5 mg or 10 mg of seladelpar once-daily. A planned interim analysis of the first 24 patients enrolled in these two dose groups demonstrated after 12 weeks of treatment a significant AP reduction from baseline of 39% and 45% for the 5 mg and 10 mg groups, respectively. On seladelpar, 45% of patients in the 5 mg and 82% of patients in the 10 mg dose groups had AP values < 1.67 times the upper limit of normal (ULN). AP is an established surrogate marker of disease progression in PBC, and reaching a level of < 1.67 x ULN is a key component in the composite endpoint used for regulatory approval.
Alongside substantial reductions in AP, patients in both dose groups experienced decreases in other liver markers of cholestasis including gamma glutamyl transferase and total bilirubin. Seladelpar also improved metabolic and inflammatory markers with patients experiencing decreases in low-density lipoprotein-C and high sensitivity C-reactive protein.
There were no serious adverse events and no safety transaminase signal was observed at either dose. Instead, mean transaminase levels decreased over the course of treatment, further supporting seladelpar’s anti-inflammatory activity. Consistent with prior studies, there was no signal for drug-induced pruritus.
After sharing preliminary results from the study, the FDA has agreed to allow continuation of seladelpar treatment beyond six months for the 5 mg and 10 mg doses.
“The data emerging from this study are impressive and support our hypothesis that lower doses of seladelpar than previously studied retain strong efficacy without raising a concern with transaminase elevations. We also see that seladelpar activity is not associated with drug-induced itch, an important benefit for patients with PBC. If these results are maintained over longer periods, we think that seladelpar could offer patients significant advantages over existing treatments,” said Professor Gideon Hirschfield, Centre for Liver Research, University of Birmingham, UK.
Dr. Pol Boudes, Chief Medical Officer of CymaBay added, “We’d like to thank the investigators and their staff as well as the patients and their families for their tremendous support. These interim results support the potent anti-cholestatic and anti-inflammatory effects of seladelpar. We are particularly excited about the FDA’s decision to allow dosing of seladelpar beyond six months enabling us to turn our attention towards planning the Phase 3 program.”
“The clinical and regulatory progress to date represent meaningful advancement in the development of seladelpar for patients with PBC,” said Sujal Shah, Interim President and CEO of CymaBay. “We are very encouraged by these results and the potential for seladelpar to improve treatment alternatives in PBC and other chronic liver diseases.”
Read the full results of the “Seladelpar Interim Data Phase 2 Low Dose Study in PBC” by
clicking here
 Obeticholic acid (Ocaliva) has recently been licenced in Canada for the treatment of people with PBC who have not fully responded to, or who are unable to tolerate, Ursodeoxycholic acid. You may have seen a recent news report from the U.S. regarding some serious adverse outcomes for PBC patients whose prescribed dose of Ocaliva was significantly higher than that recommended and approved by the FDA. This appears to be an issue primarily among patients who have advanced cirrhosis, some of whom have experienced liver injury, liver failure, and even death. We feel it’s important to make sure you are aware of this issue. If you are being treated with Ocaliva and have any questions or concerns about the appropriate dosage level for your stage of PBC, you should talk to your doctor.
Obeticholic acid (Ocaliva) has recently been licenced in Canada for the treatment of people with PBC who have not fully responded to, or who are unable to tolerate, Ursodeoxycholic acid. You may have seen a recent news report from the U.S. regarding some serious adverse outcomes for PBC patients whose prescribed dose of Ocaliva was significantly higher than that recommended and approved by the FDA. This appears to be an issue primarily among patients who have advanced cirrhosis, some of whom have experienced liver injury, liver failure, and even death. We feel it’s important to make sure you are aware of this issue. If you are being treated with Ocaliva and have any questions or concerns about the appropriate dosage level for your stage of PBC, you should talk to your doctor.



 Novartis has
Novartis has  The American Association for the Study of Liver Diseases published findings that the ASK1 Inhibitor Selonsertib reduced liver fibrosis in stage 2-3 liver fibrosis. In late 2016 Gilead announced that Selonsertib was being tested with “
The American Association for the Study of Liver Diseases published findings that the ASK1 Inhibitor Selonsertib reduced liver fibrosis in stage 2-3 liver fibrosis. In late 2016 Gilead announced that Selonsertib was being tested with “

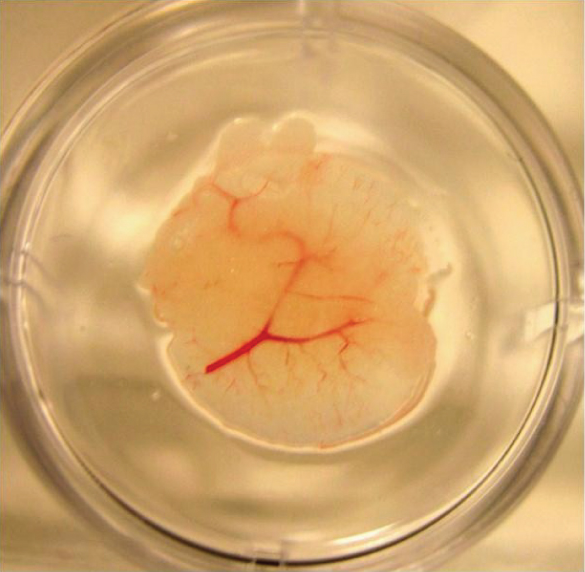 More exciting news in Liver Research. Engineered liver tissue could help millions of people who suffer from chronic liver disease but don’t qualify for a liver transplant. –
More exciting news in Liver Research. Engineered liver tissue could help millions of people who suffer from chronic liver disease but don’t qualify for a liver transplant. – 
 “Genkyotex Initiates Patient Enrollment into Phase 2 Trial of GKT831 in Primary Biliary Cholangitis” –
“Genkyotex Initiates Patient Enrollment into Phase 2 Trial of GKT831 in Primary Biliary Cholangitis” –